
Bence Szabó Gál
Professional leader
(The original, long version of the article is available by clicking here.)
What if you found out that plain old vitamin K1 was just as good, maybe even better than the expensive K2, but it happens to be the best-kept secret? Are you familiar with Dumas' Iron Mask story, in which one of two twin princes is hidden in an iron mask while the other reigns supreme? Well, the situation with vitamin K is not unlike that: K1's iron mask is that it only regulates blood clotting and is well hidden from view. When found, it is claimed to be the result of some ill-conceived study... K1 has produced the effects of K2 in both animal and human studies, and in comparisons, it has beaten K2 hands down, yet we never hear about it... Nor do we hear that the modern western diet is actually very rich in K2 due to the consumption of processed industrial meat products, while the diet of indigenous peoples and our ancestors was practically devoid of K2, but had up to 100 times as much K1... Western people's K1 consumption has been reduced to a fraction and K2 has increased steeply in comparison to indigenous peoples and our ancestors...
Contrary to custom, I'll start my article with the summary and conclusion (practical recommendation), as I presume many people might not want to wade through the tedious details... Those who get curious about the details after the summary may continue reading under the summary table...
Summary and practical recommendations:
In principle, 500-1000 mcg of K1 of moderate to good absorption per day seems sufficient in the long run. For vegetable-derived K1, this means it should be eaten with sufficient fat and preferably heat-treated, while from supplements, an oil-soluble or emulsified form should be taken with meals so that absorption is around 80% and the half-life is rather long.
Some medicines (statins, bisphosphonates, coumarin derivatives) inhibit the conversion of K1 and MK-7 to K2 MK-4, so it is advisable to take MK-4 as well, at least 1500 mcg per day (after consulting your doctor.) D3, zinc, and magnesium enhance the conversion of K1 to K2. Vitamin A (retinol) and vitamin B6 also assist the effect of K2. It is worth minding sufficient consumption of these.
To preserve the natural MK4/K1 ratio in our tissues and thus enhance the effect of vitamins K and prevent counterproductivity, if you take K2 MK-7, you should also take at least 3x as much K1, while if you take MK-4 you should also take at least the same amount of K1 so that K2 vitamins do not cause any problems.
K1 is safe on its own, and unless its conversion is blocked by these drugs, K1 is the most potent of the K vitamins. Ideally, I recommend taking at least 500mcg of vitamin K1 daily in oil-soluble or emulsified form, but preferably 1000mcg. In addition to this, I also recommend optionally taking 100-200mcg of K2 MK-7, but no more. It may even be worth taking over 1000mcg of vitamin K1. 5mg a day seems ideal as in one study this amount (taken for 2-4 years) reduced cancer risk by a quarter and fracture risk by half.
| vitamin K1 (Phylloquinone) | K2 MK-4 (Menaquinone-4, or menatetrenone) | K2 MK-7 |
Other LC-MKs (MK-5-14) |
|
|---|---|---|---|---|
| Average Western consumption /day | 50-100 mcg | 20-100 mcg (mainly from processed industrial meat products) | 0-5 mcg (Japan: ~50 mcg) | 20-1000 mcg (mainly from processed industrial meat products) |
| Average content in health-conscious nutrition/day | 200-1000 mcg (from vegetables, olives, seeds and nuts, liver) | 5-10 mcg (low content in naturally raised meats) |
0-10 mcg (Japan: ~100 mcg) |
0-10 mcg (fermented vegetables and cheese, none in naturally raised meats) |
| Maximum consumption achievable with today’s foods and reasonable effort | Ca. 2500 mcg | Ca. 100 mcg (industrial meat products) | 200 mcg (natto) | 2000 mcg (industrial meat products) |
| Consumption in native people, as well as estimated consumption in the course of human evolution during the neolithic/ day |
Native peoples: ca. 1000 mcg or more Our ancestors: 1000 mcg – 1 million mcg |
10-100 mcg | Zero | Zero, unless consuming feces regularly |
| Absorption/biological utilization from whole foods | 10-80% (increased by cooking and addition of fats and oils | zero below 600 mcg, and around a third of that of K1 above 600 mcg | 75-100% | Presumably comparable to MK-7 |
| Absorption / biological utilization dissolved in oil or from emulsified nutritional supplements taken with fats and sufficient food | ~80% | zero below 600 mcg, and around a third of that of K1 above 600 mcg | 75-100% | Presumably comparable to MK-7 |
| Half-life if taken with food | ca. 6-7 hours | ca. 4 hours | Primary half-life 6-7 hours, secondary half-life 2 days | Similar to MK-7, but with an even longer secondary half-life |
| Occurrence and ratio in animal and human tissues | All tissues contain it. Bone, heart, and liver contain primarily K1. | Most tissues contain it. The brain, kidneys, arteries, and testes contain primarily MK-4 | Not naturally occurs in tissues, but the liver may store some. | Doesn't naturally occur in tissues, but the liver may store some. |
| Relative content in the body | Ca. 10-30% (the reason it is quite so low is that it is mostly converted to K3 in the course of absorption already, and then to MK-4 in the tissues. | Ca. 70-90% (All K vitamins are converted to MK-4 in the tissues, except for a contingent left purposefully in the form of K1) |
Ca. 0% (Unnecessary, therefore the body converts it to K3, then MK-4 for effectiveness)
|
Ca. 0% (Unnecessary, therefore the body converts it to K3, then MK-4 for effectiveness) |
| Is a large dose capable of upsetting the vitamin K balance in the tissues (K1/MK-4) | No | Slightly | Yes | Yes |
| How effectively does it activate clotting factors in the liver (there is no such thing as „overactivation", the goal is to activate all of it | Very well (ca. 100 mcg are sufficient for complete activation) | Capable of activation, but ineffective | Very well (ca. 30-40 mcg suffice for full activation | As well as MK-7, if not better |
| Effectivity in activating osteocalcin (a measure of the positive effect on the bones) | Very good (just 250 mcg activate nearly fully, 500-1000 mcg completely) | No effect below 600 mcg. 1500 mcg activate well, but even 45 thousand mcg MK4 do not activate as fully as 1000 mcg K1 | 90 mcg activate somewhat, but even high doses do not reach the effect of K1. | Not studied in humans |
| Effectivity in activating MGP (a measure of the positive effect on the cardiovascular system) | Very good: 500 mcg activate nearly fully (the highest activation measured) | No trials | Good, but surpassed by K1 | Unstudied |
| Effectivity in overall immune system benefit in epidemiological studies (more specifically in lowering all-cause mortality) | Yes (Over 400 mcg) | None (Only in studies that proved invalid) | None (Only in studies that proved invalid) | None (Only in studies that proved invalid) |
| Effectivity preventing CVD/CAC/CHD observed in epidemiological studies | Yes (over 400 mcg) | None (Only in studies that proved invalid) | None (Only in studies that proved invalid) | None (Only in studies that proved invalid) |
| Positive effect on the bones in epidemiological studies | Yes (over 400 mcg) | None | None (Only in studies that proved invalid) | None (Only in studies that proved invalid) |
| Positive effect on the bones proven in placebo-controlled studies | Yes, considerable (effects have been shown at doses ranging from 100-200 mcg, 500, 1000 to 5 thousand mcg) | Yes, considerable (At doses starting at 1500 mcg, but more so at doses around 45 thousand mcg) | Yes, in 100 and 180 mcg doses | Unstudied |
| Positive effect on the cardiovascular system proven in placebo-controlled studies | Yes, it was found to be effective in all RCT studies where it was examined. (3/3) | Not (3/0), or hardly effective: in a single study, it was found mildly effective, but only in mega doses in individuals gravely vitamin-K-deficient | Not really, in a subgroup of 1 study in a 180 mcg-os dose it was slightly effective, but not for the whole group, therefore ultimately ineffective in all studies | Unstudied |
| Placebo-controlled positive effect on coronary artery calcification | Yes, effective in multiple studies | Ineffective in all studies | Ineffective in all studies, in fact, it proved harmful in the most recent study at 360 mcg per day, as it increased calcification as compared to the placebo.s | Unstudied |
| Considerable effect in lowering cancer risk in placebo-controlled studies | Yes, taking it for 4 years at 2 mg per day lowered the risk of cancer by 75% | Yes, but only at doses exceeding 45 mg. | No | No |
| Effectiveness in preventing the progression/development of liver cancer proven in human interventional studies | Yes | Yes | No | |
| Ability to decrease the size of liver cancer in human interventional studies | Yes (50 participants, ca. 80% were stabilized or got better, tumor size decrease induced in many…) | No (though full remission was achieved in a study on leukemia patients) | No | No |
| Effectiveness when taken in nutritional doses? |
Yes |
No | Yes, but it appears counterproductive above 360 mcg (though doses over 200 mcg aren't realistic for normal nutrition, anyway) | Unstudied, but presumably similar to MK-7 once more. |
| Did they ever find counterproductive effects from its consumption in any human studies? | No, never. | Yes, in human as well as in preclinical studies. | Yes, in human as well as in preclinical studies. | Their effectiveness was never studied. |
| Overall effectiveness and safety. | Very effective on all points. Very safe at any dose. | Very effective for the bones, but not so much for the cardiovascular system. Safe, though the truly effective doses of 45 thousand mg/day have raised slight concern. | Effective for the bones, not so much for the cardiovascular system. Effective and fully safe up to a dosage of 200 mcg. 360 mcg are less effective and may be harmful. | Never studied, but may be similar to MK-7. |
| Prive of an effective dose | Pennies | Expensive |
Somewhat expensive |
As good as free. |
| Price /mg | 1x | 10x | 100x | 0,1x |
| Permitted in the EU? | Yes | No | Yes | No |
| Do statins, coumarins, and bisphosphonate-type medications inhibit its effect? | Yes | No | Yes | Yes |
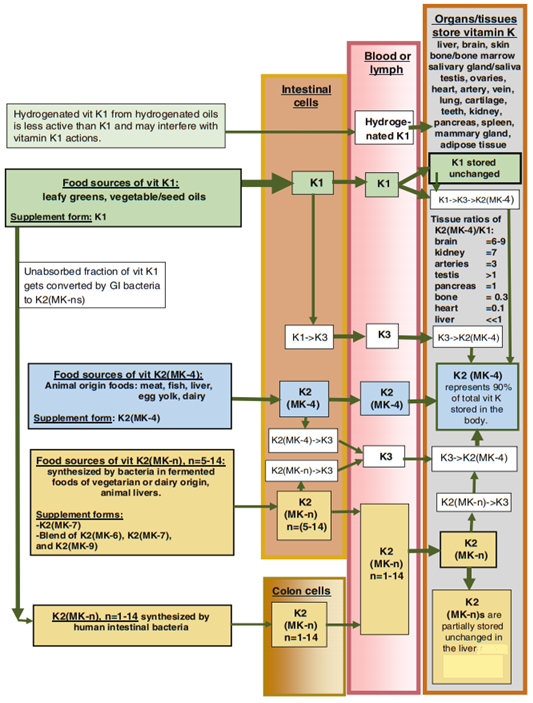
An excellent illustration of the transformation of vitamin K in the body from the chapter on vitamin K in the Pharmacology of Natural Medicines, 5th edition 2020 of the Textbook of Natural Medicines:
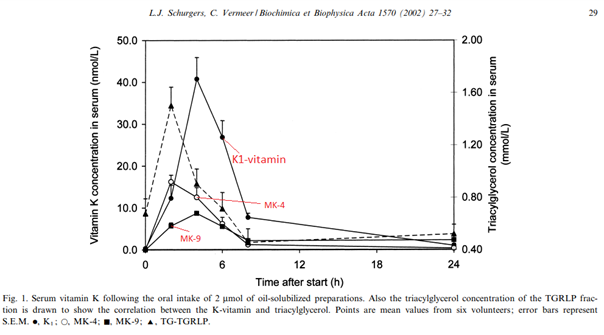
And a great chart showing the absorption of 900 mcg K1 + 900 mcg MK-4 + 1600 mcg MK-9 in a healthy person when consumed dissolved the butter of a breakfast toast:
Forms of vitamin K and their occurrence in nutrition
Vitamin K3 (menadione) - While it is not found in food, all vitamin K, whether K1 or K2, is partially or fully broken down in the body to K3, which is then converted to K2. It is therefore a transitional form not used in food products. Yet, as it is particularly effective in increasing the body's vitamin K2 levels and is inexpensive, it is used in megadoses in the diets of industrially reared animals, and in normal doses in other diets such as dog food. The vitamin K2 content of meat and liver can be high in meat products, particularly in processed meat products, because of the mega-dose of K3 in their diet. Extremely high doses of K3 worsen the antioxidant status and for this reason, it is not approved for use in supplements for humans, which is a pity because even small doses are very good at increasing the body's K2 levels.
Vitamin K1 has one type which is phylloquinone, also known as phytomenadione.
Only one form of Vitamin K2 is found naturally in animals and humans in significant amounts, menaquinone-4 (MK-4). Very small amounts of longer-chain menaquinones can also be found in our liver, which are formed by intestinal bacteria mainly from vitamin K1 and stored in the liver. One of these is menaquinone-7 (MK-7). Various bacteria also produce all lengths between MK-5 and MK-14, conceivably MK-1/2/3 and menaquinone above MK-14, too.
Vitamin K1 is abundantly found in plant foods, but also in animal foods, only to a lesser extent. K2 practically does not occur in food except for aged cheeses, fermented soy (natto), and foods made from animals fed with mega-doses of vitamin K3-enriched food, in everything else it is found only in trace amounts.
Approach to the various forms of Vitamin K based on the diet of our ancestors and the people from natural tribes
Vitamin K1 occurs abundantly in the diet of people from natural tribes (on average approx. 1000 mcg per day). In addition, during our decisive "aquatic ape" evolutionary phase that took place on the East African ocean coast, for millions of years we could also eat "sea vegetables" with an extremely high vitamin K1 content, from which we could obtain up to 1 million mcg/day of K1 (in addition, they also contain high amounts of DHA). Whether it happened or not, in today's modern diet, our intake of vitamin K1 has decreased by more than a tenth, compared to what is typical for natural people. On the other hand, our intake of vitamin K2 has increased by about 10-100 fold, because food sources of K2 have practically only existed for a few hundred years: by far the richest sources are cheeses, fermented soy, but especially modern processed and other meat products. K2 content above 10 mcg/100 g has not yet been measured in any part of free-range and wild animals, only 1-2 mcg/100 g is typical. High K2 content in animal food was measured only where, compared to the animal's natural diet, they received approx. 100x times the amount of vitamin K in their food (in this case, poor-quality food is enriched with synthetic Vitamin K3 in mega-doses, as this is a much cheaper solution than feeding them normally) and possibly, in addition, due to the poor housing, eating feces may also be common for these animals. (Feces have a very high K2 content if vitamin K intake is high and thus eating feces further increases the K2 level in meat and all tissues). According to a recent measurement, foods made from such industrially reared pigs contain 300-500 mcg of K2/100 g, while their fresh meat also contains up to 100 mcg of K2, mainly in the form of MK-8/9/10/11, which are dominant in the feces. The liver pâté made from goose liver stuffed with K3-enriched corn also contains nearly 400 mcg of K2, but in the form of MK-4 (a stuffed goose does not regurgitate its feces), while its leg meat also contains 30 mcg of MK-4 (60 mcg was also measured in chicken legs /100 g). The practice of mega-dosing K3 in the meat industry thus results in high K2 levels in modern industrially produced meat products. Presumably, if the domesticated animal does not re-eat another animal's or its own feces very often, high levels of K3 supplementation will result in high MK-4 content in the meat, while if the animal eats feces frequently, it will get so much MK-8-11 with a very long half-life, that they could surpass the level of MK-4 in the meat.
Anyway, it seems pretty clear that no food that existed thousands of years ago could really have had a K2 content above 10 mcg/100 g, unless we consumed feces (e.g. the Hadza tribe sometimes make a soup from animal feces boiled with starchy tubers and spices). Apart from fecal consumption, however, we only had access to K2 in the form of MK-4, and it is unlikely that we would have been able to approach a daily intake of 100 mcg K2, which counts as nothing because MK-4 has no effect in doses below 600 mcg and presumably it doesn't even reach our bloodstream in such low amounts. The Yupik Inuit, who eat very little plant food and live almost exclusively on animal food, have been found to be particularly deficient in vitamin K, often not even having enough vitamin K in their bodies for the proper functioning of their blood clotting factor
In summary, the consumption of Vitamin K1 by modern humans has decreased to a fraction of our evolutionary intake, while the consumption of K2 has increased many folds.
This may also be surprising because the body of humans and animals contain much more MK-4 than K1, and contains no other form of K2. It follows that a lot of K1 alone is enough to ensure the body's optimal K2 level. How could this be?
Regardless of what type of vitamin K we swallow, a significant part of it is already transformed into K3 in the intestinal cells during its absorption (even if we consumed MK-4, it is also transformed into K3 first in part or in whole). The K3 thus created from K1 and K2 reaches the tissues via the bloodstream, where an enzyme called UBIAD1 converts them into K2 MK-4 (the K3). The portion that is not transformed into K3, reaches the tissues in an unchanged form and is incorporated as such, and later converted by the tissue into K3 and only after into MK-4 according to its own needs, but this process happens much more slowly since the UBIAD1 enzyme can convert K1 into K3, but it excels in the K3->MK-4 conversion. In addition, the liver can also convert K1 into K2 MK-4 (this is enhanced by vitamin D3). Moreover, in the case of K1, K2 is also formed from the unabsorbed part! Intestinal bacteria convert nearly 100% of vitamin K1 into K2 (MK-8/9/10, etc.) measured in modern western people, although not much of it gets back into the bloodstream beyond the liver, but this is presumably partly due to its low concentration -> with a higher K1 intake, the concentration can be increased and thus more K2 can be returned.
The bottom line is that many known mechanisms ensure the Vitamin K1->K2 conversion in humans and animals. Quite a few studies have been carried out, from which the conclusion can be drawn that the K1->K3->MK-4 conversion is efficient in both humans and mice, as is the production of K1->K2 (MK-8,9, etc.) in the intestines. In a study done in healthy people, they tried to establish that approximately how much can be converted from a high dose of K1 taken orally to K3 and then to K2 within 24 hours after its absorption and they came to the conclusion that 8-30% of the dose taken, which if we use the absorbed dose it means an approximately 50% absorption rate, which does not take into account that it is also transformed later on in the tissues and by intestinal bacteria
Tissues have their own ideal vitamin K ratio (MK-4/K1). In them, Vitamin K1 can be transformed into K2 (MK-4), but there are tissues that do not transform all of it because they prefer K1. Since K2 cannot be converted back to K1, excessive doses of K2 can disrupt the ratio, while K1 cannot. K1 increases the K2 level in the tissues to the same extent as K2 MK-4 itself, while maintaining the ideal MK-4/K1 ratio characteristic of the specific tissues
Utilization
The utilization of K2 MK-7 is the best, as almost the entire dose is fully utilized, while a maximum of 80% of vitamin K1 is. In doses below 600 mcg, MK-4 is probably not useful at all, while when taken in a dose of 1000 mcg dissolved in butter, it is utilized by a third as much as K1 consumed in the same manner. MK-4 is cleared from the blood the fastest (its half-life is approximately 4 hours), while the half-life of K1 and K2 MK-7 and MK-9 dissolved in oil and taken with food is approximately 6-7 hours, however, in the case of MK-7 and MK-9, there is a secondary half-life, i.e. after its level drops to half or a quarter, it only halves every 2 days. All this means that the effect of 200 mcg MK-7 corresponds to approximately 600 mcg of K1 and 1800 mcg of K2 to MK-4 if we accept that both K1 and MK-7 are able to transform into MK-4 as needed (K1 does not need to be all transformed, since the tissues also need K1, not only MK-4, but it is important for MK-7 to transform fully and that is exactly why there can be a problem if we take too much K2 without adequate K1)
If all that I have written so far is true, then studies conducted with K1 should show a similar or better health improving and disease risk-reducing effect than those with K2, especially in studies directly comparing K1 with K2. As you will find out this is exactly the case! As they say, the proof of the pudding is in the eating, but before that let's take a closer look at what studies are used to show that K1 is not effective:
- In-vitro studies: There is no need to even comment on this since K1 can only turn into K2 in a living organism..
- Rotterdam study and others: These are epidemiological studies, i.e. they are only able to show correlation, not a cause and effect relationship. Some of these studies found that those with the highest consumption of gourmet cheese on a food questionnaire test (source K2) had lower rates of certain cardiovascular risks than those with the lowest consumption of cheese. The difference was 14mcg K2 in their diet (7mcg MK-9 + 7mcg MK-4). The group consuming the least K1 consumed about 150 mcg of K1, while those consuming the most consumed about 300 mcg. More K1 only reduced the risk less than K2, which is why these studies are often referred to, however, from 300 mcg of K1, which is not taken as an oil-based supplement, but for example, as vegetables, only a little more is absorbed than what the liver needs, so it is not surprising that they did not find a big difference in the effect of very low and extremely low vitamin K1 consumption. What is more surprising is what is in these gourmet cheeses, or is it just that gourmets eat far less junk food? Since 2016, however, we have known that the consumption of processed meat products is associated with up to 10-20x as much K2 intake as the high consumption of cheese, but until now it was considered close to zero. For this reason, all similar K2 studies, where the K2 content of the industrial meat products was considered to be close to 0, can either be thrown in the trash or recalculated afterward. And all studies are like that. Later, in a more precisely organized study in Greece, it was found that in the participants with a higher (average 600 mcg) K1 intake, more types of cardiovascular problems were halved than in the Rotterdam study, moreover, the risk of cancer and total mortality was also significantly reduced ... And here K2 was examined simultaneously with K1, not just separately. Higher K2 intake was not found to be effective, even though it was higher than in the Rotterdam study and there were bigger differences, although this does not mean anything, since the high K2 content of meat products was not taken into account in this study either, so the corresponding statement regarding K2 is also incorrect here, however, the one regarding K1 is correct, since the K1 intake was sufficiently high, from which enough reached the tissues beyond the activation of the blood coagulation factors.
The conversion of K1 to K2 is inhibited by certain medications such as statins and bisphosphonates. It is also possible to point out that certain trials were conducted while taking such drugs, and in these cases, the competitor's leg was broken before the competition: They also used to point to such a mouse test, where the researchers simply wanted to prove that K1 must be able to transform into K2 (MK-4) to exert its anti-vascular calcification effect. Here, such a conversion blocking drug (Warfarin) was administered throughout the study, while the mice were given K1 or K2 MK-4 for months. K2 (MK-4) prevented vascular calcification, while K1 did not. That is, if K1 is not allowed to transform into K2 MK-4, then it has no effect. (It was not tested, but I am sure that MK-7 would not have had an effect either, since it also has to be transformed into MK-4). Later, in another study, mice with severe aortic and coronary atherosclerosis were given high doses of K1 or K2 (MK-4) without the conversion-blocking drug (about 50 mg/day in human terms). Both reversed and approximately halved the calcified plaque deposits in 6 months time. K1 was significantly more effective than MK-4, which may be because as they have measured in the tissues of the animals, MK-4 administration upset the ideal MK4/K1 ratio, while K1 administration maintained it while increasing tissue MK-4 by the same amount, as MK-4 itself.
Human trials:
After we have cleared the contradictions, let's look at what's most important: are these effects confirmed by human interventional studies
Their osteocalcin and MGP activating effect: The effect on the heart/vascular system, calcification, teeth, and skin depends on how well a type of vitamin K is able to activate MGP, while the effect on the skeletal system and teeth depends on how well they activate osteocalcin. All of these were activated best by K1 in human clinical trials and at a dose of only 500-1000 mcg. Similar good results have not yet been achieved with any amount of K2, the closest was once with 45,000 mcg of K2 (MK-4)...
Epidemiological studies have already been mentioned in connection with the Rotterdam study, but they are not even worth mentioning, as they are completely irrelevant, because they were calculated using wrong data, as it turned out a few years ago...
In placebo-controlled studies looking at the effect on bones, doses of vitamin K1 between 500 mcg and 5 mg were similarly effective as 45 mg (=45 thousand mcg) doses of MK-4. MK-7 was effective at 100-180 mcg, although significantly less so than K1 or MK4. MK-7 was not, but both K1 and K2 were able to not only slow/stop, but also reverse osteoporosis. There are several studies on MK-4, but all of them were 45 mg doses, while the highest dose of K1 was 5 mg, which was studied at length on the bones. The fact that K1 is just as effective and sometimes more effective than K2 MK-4 even at such a lower dose can be explained not only by maintaining the correct tissue ratio but also by its better absorption
In examining the effects on the cardiovascular system, K1 turned out to be the most effective in doses between 0.5 and 5 mg, although MK-4 also had a positive effect in doses of 45 mg, but to a lesser extent. MK-7 has not been found in any studies to prevent or improve calcification or any other cardiovascular problem. In fact, in the most recent study, where the most accurate measurement method to date was used to determine calcification, they found that taking 360 mcg of K2 MK-7 increased calcification in blood vessels by 10% compared to placebo, instead of decreasing it. (In the case of MK-7, this is already an impractically high dose, because even people with the highest dietary MK-7 intake in the world consume less than 200 mcg per day on average, and knowing its slow half-life, such a dose accumulates when taken daily and can disrupt the tissue MK4/K1 ratio)
Head-to-head comparison
Of course, they can only be properly compared if they are examined head-to-head in the same study, in the same setting, and randomized between the same subjects. Fortunately, there are 2 such studies, after which they were never done again, as it is financially very painful for the manufacturers of Vitamin K2, that as it turned out the 100 times cheaper vitamin K1 is more effective than the expensive K2... In one of these studies, the effect of 45mg of K2 MK-4 per day was compared with 1mg of vitamin K1 per day. They performed equally well, and K1 even had a slight edge (both prevented BMD changes, but 1mg K1 activated osteocalcin a little more than 45mg K2
In another study, only 100 mcg of K1 or K2 MK-7 was added to a diet that already contained 100-150 mcg of K1. K1 significantly increased the bone density of the lower part of the spine (3x more) than K2, while their other effects were the same, for example, they both reduced soft tissue calcification.
Finally, it is also worth mentioning the studies done on cancer: Vitamin K3 has the strongest anti-tumor effect, which is converted from all types of vitamin K in a similar manner. For this reason, also K1 seems to be the most practical and indeed the best here, since it is the cheapest, has at least 3x better absorption than K2 MK-4, and although MK-7 is 3x better absorbed than K1, it is 100x more expensive, and its 360 mcg dose can already be risky while mg doses would be needed in this case.
In one study, 440 post-menopausal women took 5 mg of K1 or placebo daily for 4 years. This reduced the incidence of cancer to only a quarter of that of the placebo group, while also halving the number of bone fractures! This is the only placebo-controlled study on the relationship between K1 and cancer. This is roughly the same as the effect that has been observed in several studies with doses of 45mg or higher of MK-4 per day
In addition, there is also 1 study of K1 and K2 MK-4, where their doses of 40-45mg or higher caused a reduction in tumor size or even complete remission. In one K1 study, with approximately 50 subjects stabilization of the condition was achieved in 75% of them, while tumor size reduction was achieved in 15%. These were end-stage, metastatic, inoperable cases of liver cancer, so this is a surprisingly good result.
[The cover image of the note shows natto. 100 grams contains 900 μg of vitamin K, which is 9 times the recommended daily intake value.]
- Saiko IKEDA, Fumiaki HANZAWA, Saki TAKAHASHI, Norie SUZUKI, Kana SANO, Hiroaki ODA, Tomono UCHIDA, Tissue Distribution of Menaquinone-7 and the Effect of α-Tocopherol Intake on Menaquinone-7 Concentration in Rats, Journal of Nutritional Science and Vitaminology, 2018, Volume 64, Issue 6, Pages 391-398, Released December 31, 2018, Online ISSN 1881-7742, Print ISSN 0301-4800, https://doi.org/10.3177/jnsv.64.391
- Jin S, Sell JL. Dietary vitamin K1 requirement and comparison of biopotency of different vitamin K sources for young turkeys. Poult Sci. 2001 May;80(5):615-20. doi: 10.1093/ps/80.5.615. PMID: 11372711.
- Open access peer-reviewed chapter Vitamin K2 in Animal Health: An Overview By Jayde O’Neil, Bethany Scarrott, Ragnhild Aven Svalheim, Jonathan Elliott and Stephen J. Hodges Submitted: January 15th 2016Reviewed: April 22nd 2016Published: March 22nd 2017 DOI: 10.5772/63901 https://www.intechopen.com/books/vitamin-k2-vital-for-health-and-wellbeing/vitamin-k2-in-animal-health-an-overview
- Open access peer-reviewed chapter Vitamin K2 in Animal Health: An Overview By Jaydn.com/books/vitamin-k2-vital-for-health-and-wellbeing/vitamin-k2-in-animal-health-an-overview
- Hirauchi, K., Sakano, T., Notsumoto, S., Nagaoka, auTadayoshi, Morimoto, A., Fujimoto, K., … Suzuki, Y. (1989). Measurement of k vitamins in animal tissues by high-performance liquid chromatography with fluorimetric detection. Journal of Chromatography B: Biomedical Sciences and Applications, 497, 131–137. doi:10.1016/0378-4347(89)80012-x
- https://www.fda.gov/animal-veterinary/safe-feed/vitamin-k-substances-and-animal-feed
- Schurgers LJ, Vermeer C. (2000) Determination of phylloquinone and menaquinones in food. Effect of food matrix on circulating vitamin K concentrations. Haemostasis. 30(6):298-307
- Koivu-Tikkanen TJ, Ollilainen V, Piironen VI. (2000) Determination of phylloquinone and menaquinones in animal products with fluorescence detection after postcolumn reduction with metallic zinc. Journal of Agriculture and Food Chemistry. 48(12):6325-31.
- Rødbotten, R., Gundersen, T., Vermeer, C., & Kirkhus, B. (2014). Vitamin K2 in different bovine muscles and breeds. Meat Science, 97(1), 49–53. doi:10.1016/j.meatsci.2014.01.005
- Hirauchi, K., Sakano, T., Notsumoto, S., Nagaoka, auTadayoshi, Morimoto, A., Fujimoto, K., … Suzuki, Y. (1989). Measurement of k vitamins in animal tissues by high-performance liquid chromatography with fluorimetric detection. Journal of Chromatography B: Biomedical Sciences and Applications, 497, 131–137. doi:10.1016/0378-4347(89)80012-x
- https://honey-guide.com/2014/03/10/menaquinones-k2-and-phylloquinone-k1-content-of-animal-products-and-fermented-foods
- Fu, X., Shen, X., Finnan, E. G., Haytowitz, D. B., & Booth, S. L. (2016). Measurement of Multiple Vitamin K Forms in Processed and Fresh-Cut Pork Products in the U.S. Food Supply. Journal of Agricultural and Food Chemistry, 64(22), 4531–4535. doi:10.1021/acs.jafc.6b00938
- J Philip Karl, Mohsen Meydani, Junaidah B Barnett, Sally M Vanegas, Kathryn Barger, Xueyan Fu, Barry Goldin, Anne Kane, Helen Rasmussen, Pajau Vangay, Dan Knights, Satya S Jonnalagadda, Edward Saltzman, Susan B Roberts, Simin N Meydani, Sarah L Booth, Fecal concentrations of bacterially derived vitamin K forms are associated with gut microbiota composition but not plasma or fecal cytokine concentrations in healthy adults, The American Journal of Clinical Nutrition, Volume 106, Issue 4, October 2017, Pages 1052–1061, https://doi.org/10.3945/ajcn.117.155424
- Au, Nicholas T et al. “Dietary Vitamin K and Association with Hepatic Vitamin K Status in a Yup’ik Study Population from Southwestern Alaska.” Molecular nutrition & food research vol. 62,3 (2018): 10.1002/mnfr.201700746. doi:10.1002/mnfr.201700746
- Rødbotten, R., Gundersen, T., Vermeer, C., & Kirkhus, B. (2014). Vitamin K2 in different bovine muscles and breeds. Meat Science, 97(1), 49–53. doi:10.1016/j.meatsci.2014.01.005
- Schurgers LJ, Vermeer C. (2000) Determination of phylloquinone and menaquinones in food. Effect of food matrix on circulating vitamin K concentrations. Haemostasis. 30(6):298-307
- Eaton SB, Eaton SB 3rd, Konner MJ. Paleolithic nutrition revisited: a twelve-year retrospective on its nature and implications. Eur J Clin Nutr. 1997 Apr;51(4):207-16. doi: 10.1038/sj.ejcn.1600389. PMID: 9104571. ] [Loren Cordain: The Nutritional Characteristics of a Contemporary Diet Based Upon Paleolithic Food Groups, JANA Vol. 5, No. 3 Summer 2002 https://thepaleodiet.com/wp-content/uploads/2015/08/The-Nutritional-Characteristics-of-a-Contemporary-Diet-Based-Upon-Paleolithic-Food-Groups-The-Paleo-Diet.pdf
- Simes DC, Viegas CSB, Araújo N, Marreiros C. Vitamin K as a Diet Supplement with Impact in Human Health: Current Evidence in Age-Related Diseases. Nutrients. 2020;12(1):138. Published 2020 Jan 3. doi:10.3390/nu12010138
- Thijssen HH, Drittij-Reijnders MJ. Vitamin K status in human tissues: tissue-specific accumulation of phylloquinone and menaquinone-4. Br J Nutr. 1996 Jan;75(1):121-7. doi: 10.1079/bjn19960115. PMID: 8785182.
- Fu, X., Moreines, J. & Booth, S.L. Vitamin K supplementation does not prevent bone loss in ovariectomized Norway rats. Nutr Metab (Lond) 9, 12 (2012). https://doi.org/10.1186/1743-7075-9-12
- Yamaguchi, M., Taguchi, H., Gao, Y. H., Igarashi, A., & Tsukamoto, Y. (1999). Effect of vitamin K 2 (menaquinone-7) in fermented soybean ( natto ) on bone loss in ovariectomized rats. Journal of Bone and Mineral Metabolism, 17(1), 23–29. doi:10.1007/s007740050059
- Sato T, Kawahara R, Kamo S, Saito S. Comparison of menaquinone-4 and menaquinone-7 in rats. Vitamins (Japan) 2007;81:377–381.
- Ikeda S, Hanzawa F, Takahashi S, Suzuki N, Sano K, Oda H, Uchida T. Tissue Distribution of Menaquinone-7 and the Effect of α-Tocopherol Intake on Menaquinone-7 Concentration in Rats. J Nutr Sci Vitaminol (Tokyo). 2018;64(6):391-398. doi: 10.3177/jnsv.64.391. PMID: 30606961
- Okano T, Shimomura Y, Yamane M, Suhara Y, Kamao M, Sugiura M, Nakagawa K. Conversion of phylloquinone (Vitamin K1) into menaquinone-4 (Vitamin K2) in mice: two possible routes for menaquinone-4 accumulation in cerebra of mice. J Biol Chem. 2008 Apr 25;283(17):11270-9. doi: 10.1074/jbc.M702971200. Epub 2007 Dec 14. PMID: 18083713
- Hirota Y, Tsugawa N, Nakagawa K, et al. Menadione (vitamin K3) is a catabolic product of oral phylloquinone (vitamin K1) in the intestine and a circulating precursor of tissue menaquinone-4 (vitamin K2) in rats. J Biol Chem. 2013;288(46):33071-33080. doi:10.1074/jbc.M113.477356
- Ala Al Rajabi, Sarah L. Booth, James W. Peterson, Sang Woon Choi, John W. Suttie, M. Kyla Shea, Benchun Miao, Michael A. Grusak, Xueyan Fu, Deuterium-Labeled Phylloquinone Has Tissue-Specific Conversion to Menaquinone-4 among Fischer 344 Male Rats, The Journal of Nutrition, Volume 142, Issue 5, May 2012, Pages 841–845, https://doi.org/10.3945/jn.111.155804 – Megj.: „In conclusion, MK-4 is the predominant form of vitamin K in multiple tissues, but there appears to be a tissue-specific regulation for the conversion of PK to MK-4. To convert PK to MK-4, the phytyl side chain in PK is removed and a geranylgeranyl side chain is added to the MD nucleus, forming MK-4. However, our data do not support the involvement of enterocytes in this conversion.”
- Nakagawa K, Hirota Y, Sawada N, Yuge N, Watanabe M, Uchino Y, Okuda N, Shimomura Y, Suhara Y, Okano T. Identification of UBIAD1 as a novel human menaquinone-4 biosynthetic enzyme. Nature. 2010 Nov 4;468(7320):117-21. doi: 10.1038/nature09464. Epub 2010 Oct 17. PMID: 20953171.
- Shearer MJ, Fu X, Booth SL. Vitamin K nutrition, metabolism, and requirements: current concepts and future research. Adv Nutr. 2012;3(2):182-195. Published 2012 Mar 1. doi:10.3945/an.111.001800 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3648719 – Megj.:„ (UBIAD1) that Nakagawa et al. (60) proposed not only catalyzed the well-described prenylation of precursor menadione to MK-4 but might also be responsible for the initial side chain cleavage of phylloquinone or other MK.”
- https://chrismasterjohnphd.com/blog/2009/04/07/tufts-university-confirms-that-vitamin
- Fu X, Wang XD, Mernitz H, Wallin R, Shea MK, Booth SL. 9-Cis retinoic acid reduces 1alpha,25-dihydroxycholecalciferol-induced renal calcification by altering vitamin K-dependent gamma-carboxylation of matrix gamma-carboxyglutamic acid protein in A/J male mice. J Nutr. 2008 Dec;138(12):2337-41. doi: 10.3945/jn.108.093724. PMID: 19022954.
- J Philip Karl, Mohsen Meydani, Junaidah B Barnett, Sally M Vanegas, Kathryn Barger, Xueyan Fu, Barry Goldin, Anne Kane, Helen Rasmussen, Pajau Vangay, Dan Knights, Satya S Jonnalagadda, Edward Saltzman, Susan B Roberts, Simin N Meydani, Sarah L Booth, Fecal concentrations of bacterially derived vitamin K forms are associated with gut microbiota composition but not plasma or fecal cytokine concentrations in healthy adults, The American Journal of Clinical Nutrition, Volume 106, Issue 4, October 2017, Pages 10521061, https://doi.org/10.3945/ajcn.117.155424
- Ikeda S, Hanzawa F, Takahashi S, Suzuki N, Sano K, Oda H, Uchida T. Tissue Distribution of Menaquinone-7 and the Effect of α-Tocopherol Intake on Menaquinone-7 Concentration in Rats. J Nutr Sci Vitaminol (Tokyo). 2018;64(6):391-398. doi: 10.3177/jnsv.64.391. PMID: 30606961.
- Okano T, Shimomura Y, Yamane M, Suhara Y, Kamao M, Sugiura M, Nakagawa K. Conversion of phylloquinone (Vitamin K1) into menaquinone-4 (Vitamin K2) in mice: two possible routes for menaquinone-4 accumulation in cerebra of mice. J Biol Chem. 2008 Apr 25;283(17):11270-9. doi: 10.1074/jbc.M702971200. Epub 2007 Dec 14. PMID: 18083713.
- Yamaguchi M, Kakuda H, Gao YH, et al. Prolonged intake of fermented soybean (natto) diets containing vitamin K2 (menaquinone-7) prevents bone loss in ovariectomized rats. J Bone Miner Metab. 2000;18(2): 71–76.
- Sato T, Kawahara R, Kamo S, Saito S. Comparison of menaquinone-4 and menaquinone-7 in rats. Vitamins (Japan) 2007;81:377–381.].
- Ikeda S, Hanzawa F, Takahashi S, Suzuki N, Sano K, Oda H, Uchida T. Tissue Distribution of Menaquinone-7 and the Effect of α-Tocopherol Intake on Menaquinone-7 Concentration in Rats. J Nutr Sci Vitaminol (Tokyo). 2018;64(6):391-398. doi: 10.3177/jnsv.64.391. PMID: 30606961.
- Craciun AM, Wolf J, Knapen MH, Brouns F, Vermeer C. Improved bonemetabolism in female elite athletes after vitamin K supplementation. Int J Sports Med. 1998;19(7):479–484. PubMed PMID: 9839845.
- Schurgers LJ, Spronk HM, Soute BA, et al. Regression of warfarin-induced medial elastocalcinosis by high intake of vitamin K in rats. Blood. 2007;109(7):2823–2831.
- Spronk HM, Soute BA, Schurgers LJ, et al. Tissue-specific utilization of menaquinone-4 results in the prevention of arterial calcification in warfarin-treated rats. J Vasc Res. 2003;40(6):531–537
- Vitamin K2 in Animal Health: An Overview By Jayde O’Neil, Bethany Scarrott, Ragnhild Aven Svalheim, Jonathan Elliott and Stephen J. Hodges Submitted: January 15th 2016Reviewed: April 22nd 2016Published: March 22nd 2017 DOI: 10.5772/63901 https://www.intechopen.com/books/vitamin-k2-vital-for-health-and-wellbeing/vitamin-k2-in-animal-health-an-overview
- Hirota Y, Tsugawa N, Nakagawa K, et al. Menadione (vitamin K3) is a catabolic product of oral phylloquinone (vitamin K1) in the intestine and a circulating precursor of tissue menaquinone-4 (vitamin K2) in rats. J Biol Chem. 2013;288(46):33071-33080. doi:10.1074/jbc.M113.477356 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3829156
- Thijssen et al: Menadione is a metabolite of oral vitamin K. British Journal of Nutrition (2006), 95, 260–266
- Thijssen HH, Drittij MJ, Vermeer C, Schoffelen E. Menaquinone-4 in breast milk is derived from dietary phylloquinone. Br J Nutr. 2002 Mar;87(3):219-26. doi: 10.1079/BJNBJN2001505. PMID: 12064330.
- Sato T, Kawahara R, Kamo S, Saito S. Comparison of menaquinone-4 and menaquinone-7 in rats. Vitamins (Japan) 2007;81:377–381.
- Koitaya, N., Sekiguchi, M., Tousen, Y., Nishide, Y., Morita, A., Yamauchi, J., … Ishimi, Y. (2013). Low-dose vitamin K2 (MK-4) supplementation for 12 months improves bone metabolism and prevents forearm bone loss in postmenopausal Japanese women. Journal of Bone and Mineral Metabolism, 32(2), 142–150. doi:10.1007/s00774-013-0472-7
- Inaba N, Sato T, Yamashita T. Low-Dose Daily Intake of Vitamin K(2) (Menaquinone-7) Improves Osteocalcin γ-Carboxylation: A Double-Blind, Randomized Controlled Trials. J Nutr Sci Vitaminol (Tokyo). 2015;61(6):471-80. doi: 10.3177/jnsv.61.471. PMID: 26875489.
- https://chrismasterjohnphd.com/blog/2009/04/07/tufts-university-confirms-that-vitamin
- Fu X, Wang XD, Mernitz H, Wallin R, Shea MK, Booth SL. 9-Cis retinoic acid reduces 1alpha,25-dihydroxycholecalciferol-induced renal calcification by altering vitamin K-dependent gamma-carboxylation of matrix gamma-carboxyglutamic acid protein in A/J male mice. J Nutr. 2008 Dec;138(12):2337-41. doi: 10.3945/jn.108.093724. PMID: 19022954.
- Funahashi N, Hirota Y, Nakagawa K, Swada N, Watanabe M, Suhara Y, Okano T. YY1 positively regulates human UBIAD1 expression. Biochem Biophys Res Commun. 2015 May 1;460(2):238-44. doi: 10.1016/j.bbrc.2015.03.018. Epub 2015 Mar 12. PMID: 25772619.
- Hirota Y, Nakagawa K, Sawada N, Okuda N, Suhara Y, Uchino Y, Kimoto T, Funahashi N, Kamao M, Tsugawa N, Okano T. Functional characterization of the vitamin K2 biosynthetic enzyme UBIAD1. PLoS One. 2015 Apr 15;10(4):e0125737. doi: 10.1371/journal.pone.0125737. PMID: 25874989; PMCID: PMC4398444.
- Schurgers LJ, Teunissen KJ, Hamulyák K, Knapen MH, Vik H, Vermeer C. Vitamin K-containing dietary supplements: comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood. 2007 Apr 15;109(8):3279-83. doi: 10.1182/blood-2006-08-040709. Epub 2006 Dec 7. PMID: 17158229
- Sato T, Kawahara R, Kamo S, Saito S. Comparison of menaquinone-4 and menaquinone-7 in rats. Vitamins (Japan) 2007;81:377–381.
- Takeuchi, A., et al. “Minimal effective dose of vitamin K2 (menaquinone-4) on serum osteocalcin concentration in Japanese subjects and safety evaluation of vitamin K2 supplemented in calcium tablet.” J Jpn Soc Clin Nutr 26 (2005): 254-260.
- Sato, Toshiro et al. “Comparison of menaquinone-4 and menaquinone-7 bioavailability in healthy women.” Nutrition journal vol. 11 93. 12 Nov. 2012, doi:10.1186/1475-2891-11-93.
- Nakamura E, Aoki M, Watanabe F, Kamimura A. Low-dose menaquinone-4 improves γ-carboxylation of osteocalcin in young males: a non-placebo-controlled dose-response study. Nutr J. 2014 Aug 27;13:85. doi: 10.1186/1475-2891-13-85. PMID: 25163392; PMCID: PMC4155127.
- Schurgers LJ, Vermeer C. Differential lipoprotein transport pathways of K-vitamins in healthy subjects. 2002; 1570: 27-32.
- Binkley NC, Krueger DC, Kawahara TN, et al. A high phylloquinone intake is required to achieve maximal osteocalcin gamma-carboxylation. Am J Clin Nutr. 2002;76(5):1055–1060.
- Knapen MH, Braam LA, Drummen NE, Bekers O, Hoeks AP, Vermeer C. Menaquinone-7 supplementation improves arterial stiffness in healthy postmenopausal women. A double-blind randomised clinical trial. Thromb Haemost. 2015;113(5):1135–1144. https://doi.org/10.1160/ TH14-08-0675. Epub 2015 Feb 19. PubMed PMID: 25694037.
- Fulton RL, McMurdo ME, Hill A, et al. Effect of vitamin K on vascular health and physical function in older people with vascular disease—a randomised controlled trial. J Nutr Health Aging. 2016;20(3):325–333. https://doi.org/10.1007/s12603-015-0619 PubMed PMID: 26892582.
- Theuwissen E, Cranenburg EC, Knapen MH, et al. Low-dose menaquinone-7 supplementation improved extra-hepatic vitamin K status, but had no effect on thrombin generation in healthy subjects. Br J Nutr. 2012;108(9):1652–1657. https://doi.org/10.1017/S0007114511007185. Epub 2012 Jan 31. PubMed PMID: 22289649.]
- Shea MK, O’Donnell CJ, Vermeer C, et al. Circulating uncarboxylated matrix gla protein is associated with vitamin K nutritional status, but not coronary artery calcium, in older adults. J Nutr. 2011;141(8):1529–1534. https://doi.org/10.3945/jn.111.139634 Epub 2011 May 31. PubMed PMID: 21628633; PubMed Central PMCID: PMC3138643.]
- Dalmeijer GW, van der Schouw YT, Magdeleyns E, Ahmed N, Vermeer C, Beulens JW. The effect of menaquinone-7 supplementation on circulating species of matrix Gla protein. Atherosclerosis. 2012;225(2):397–402. https://doi.org/10.1016/j.atherosclerosis.2012.09.019 Epub 2012 Sep 25. PubMed PMID: 23062766]
- Brandenburg VM, Reinartz S, Kaesler N, et al. Slower progress of aortic valve calcification with vitamin K supplementation: results from a prospective interventional proof-of-concept study. Circulation. 2017;135(21):2081–2083. https://doi.org/10.1161/CIRCULATIONAHA.116.027011 PubMed PMID: 28533322.]
- Spronk HM, Soute BA, Schurgers LJ, et al. Tissue-specific utilization of menaquinone-4 results in the prevention of arterial calcification in warfarin-treated rats. J Vasc Res. 2003;40(6):531–53
- Schurgers LJ, Spronk HM, Soute BA, et al. Regression of warfarin-induced medial elastocalcinosis by high intake of vitamin K in rats. Blood. 2007;109(7):2823–2831
- Fu X, Moreines J, Booth SL. Vitamin K supplementation does not prevent bone loss in ovariectomized Norway rats. Nutr Metab (Lond). 2012;9(1):12
- Yamaguchi M, Kakuda H, Gao YH, et al. Prolonged intake of fermented soybean (natto) diets containing vitamin K2 (menaquinone-7) prevents bone loss in ovariectomized rats. J Bone Miner Metab. 2000;18(2): 71–76.
- Spronk HM, Soute BA, Schurgers LJ, et al. Tissue-specific utilization of menaquinone-4 results in the prevention of arterial calcification in warfarin-treated rats. J Vasc Res. 2003;40(6):531–537.
- Schurgers LJ, Spronk HM, Soute BA, et al. Regression of warfarin-induced medial elastocalcinosis by high intake of vitamin K in rats. Blood. 2007;109(7):2823–2831
- Joyce C McCann, Bruce N Ames, Vitamin K, an example of triage theory: is micronutrient inadequacy linked to diseases of aging?, The American Journal of Clinical Nutrition, Volume 90, Issue 4, October 2009, Pages 889–907, https://doi.org/10.3945/ajcn.2009.27930
- Geleijnse JM, Vermeer C, Grobbee DE, Schurgers LJ, Knapen MH, van der Meer IM, Hofman A, Witteman JC: Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam Study. J Nutr. 2004 Nov; 134(11): 3100-3105.
- Beulens JW, Bots ML, Atsma F, Bartelink ML, Prokop M, Geleijnse JM, Witteman JC, Grobbee DE, van der Schouw YT. High dietary menaquinone intake is associated with reduced coronary calcification. Atherosclerosis. 2009 Apr;203(2):489-93.
- Erkkilä AT, Booth SL, Hu FB, et al. Phylloquinone intake as a marker for coronary heart disease risk but not stroke in women. Eur J Clin Nutr. 2005;59(2):196–204. PubMed PMID: 15454972)
- Fu, X., Shen, X., Finnan, E. G., Haytowitz, D. B., & Booth, S. L. (2016). Measurement of Multiple Vitamin K Forms in Processed and Fresh-Cut Pork Products in the U.S. Food Supply. Journal of Agricultural and Food Chemistry, 64(22), 4531–4535. doi:10.1021/acs.jafc.6b00938
- Juanola-Falgarona M, Salas-Salvadó J,et. al. Dietary intake of vitamin K is inversely associated with mortality risk. J Nutr. 2014;144(5):743–750. https://doi.org/10.3945/jn.113.187740. Epub 2014 Mar 19. Erratum in: J Nutr. 2016 Mar;146(3):653. PubMed PMID: 24647393
- Schurgers LJ, Spronk HM, Soute BA, et al. Regression of warfarin-induced medial elastocalcinosis by high intake of vitamin K in rats. Blood. 2007;109(7):2823–2831
- Fu X, Moreines J, Booth SL. Vitamin K supplementation does not prevent bone loss in ovariectomized Norway rats. Nutr Metab (Lond). 2012;9(1):12
- Binkley N, Harke J, Krueger D, et al. Vitamin K treatment reduces undercarboxylated osteocalcin but does not alter bone turnover, density,or geometry in healthy postmenopausal North American women. J Bone Miner Res. 2009;24(6):983–991
- Kanellakis S, Moschonis G, Tenta R, et al. Changes in parameters of bone metabolism in postmenopausal women following a 12-month intervention period using dairy products enriched with calcium, vitamin D, and phylloquinone (vitamin K(1)) or menaquinone-7 (vitamin K (2)): the Postmenopausal Health Study II. Calcif Tissue Int. 2012;90(4):251–262. https://doi.org/10.1007/s00223-012-9571-z Epub 2012 Mar 4. PubMed PMID: 22392526.
- Iwamoto et al.: High-dose vitamin K supplementation reduces fracture incidence in postmenopausal women: a review of the literature. Nutrition Research 29 (2009) 221–228
- Hao G, Zhang B, Gu M, et al. Vitamin K intake and the risk of fractures: A meta-analysis. Medicine (Baltimore). 2017;96(17):e6725. doi:10.1097/MD.0000000000006725
- Shea MK, Dallal GE, Dawson-Hughes B, et al. Vitamin K, circulating cytokines, and bone mineral density in older men and women. Am J Clin Nutr. 2008;88(2):356–363.
- Walther B, Karl JP, Booth SL, Boyaval P. Menaquinones, bacteria, and the food supply: the relevance of dairy and fermented food products to vitamin K requirements.
- Erkkilä AT, Booth SL, Hu FB, et al. Phylloquinone intake as a marker for coronary heart disease risk but not stroke in women. Eur J Clin Nutr. 2005;59(2):196–204. PubMed PMID: 15454972.
- Binkley N, Harke J, Krueger D, et al. Vitamin K treatment reduces undercarboxylated osteocalcin but does not alter bone turnover, density,or geometry in healthy postmenopausal North American women. J Bone Miner Res. 2009;24(6):983–991.
- Braam LA, Knapen MH, Geusens P, Brouns F, Vermeer C. Factors affecting bone loss in female endurance athletes: a two-year follow-up study. Am J Sports Med. 2003;31(6):889–895. PubMed PMID: 14623654.
- Sato Y, Nakamura R, Satoh M, et al. Thyroid hormone targets matrix Gla protein gene associated with vascular smooth muscle calcification. Circ Res. 2005;97(6):550–557.
- Kanellakis S, Moschonis G, Tenta R, et al. Changes in parameters of bone metabolism in postmenopausal women following a 12-month intervention period using dairy products enriched with calcium, vitamin D, and phylloquinone (vitamin K(1)) or menaquinone-7 (vitamin K (2)): the Postmenopausal Health Study II. Calcif Tissue Int. 2012;90(4):251–262. https://doi.org/10.1007/s00223-012-9571-z. Epub 2012 Mar 4. PubMed PMID: 22392526
- Harshman SG, Saltzman E, Booth SL. Vitamin K: dietary intake and requirements in different clinical conditions. Curr Opin Clin Nutr Metab Care. 2014;17(6):531–538. https://doi.org/10.1097/MCO.0000000000000112. Review. PubMed PMID: 25232640
- Iwamoto J, Sato Y. Menatetrenone for the treatment of osteoporosis. Expert Opin Pharmacother. 2013 Mar;14(4):449-58. doi: 10.1517/14656566.2013.763796. Epub 2013 Jan 25. Retraction in: Expert Opin Pharmacother. 2019 Aug;20(12):1531. PMID: 23346882.]
- Knapen MH, Schurgers LJ, Vermeer C. Vitamin K2 supplementation improves hip bone geometry and bone strength indices in postmenopausal women. Osteoporos Int. 2007;18(7):963–972.
- Knapen MH, Schurgers LJ, Vermeer C. Vitamin K2 supplementation improves hip bone geometry and bone strength indices in postmenopausal women. Osteoporos Int. 2007;18(7):963–972.
- Koitaya N, Sekiguchi M, Tousen Y, et al. Low-dose (1.5mg) vitamin K2 (MK-4) supplementation for 12 months improves bone metabolism and prevents forearm bone loss in postmenopausal Japanese women. J Bone
- Miner Metab. 2014;32(2):142–150. https://doi.org/10.1007/s00774-013-0472-7. Epub 2013 May 24. PubMed PMID: 23702931.
- Ushiroyama T, Ikeda A, Ueki M. Effect of continuous combined therapy with vitamin K(2) and vitamin D(3) on bone mineral density and coagulofibrinolysis function in postmenopausal women. Maturitas. 2002;41(3):211–221.
- Binkley N, Harke J, Krueger D, et al. Vitamin K treatment reduces undercarboxylated osteocalcin but does not alter bone turnover, density, or geometry in healthy postmenopausal North American women. J Bone
- Miner Res. 2009;24(6):983–991.
- Orimo H, Shiraki M, Tomita A, Morii H, Fujita T, Ohata M. Effects of menatetrenone on the bone and calcium metabolism in osteoporosis: a double‐ blind placebo‐controlled study. J Bone Miner Metab, 1998. 16: 106–112.: Megj.: napi 90 mg nem csak lassítani vagy megállítani tudta, de vissza is fordítani a csontritkulást (+2% BMD). 80 fő
- Shiraki M, Shiraki Y, Aoki C, Miura M . Vitamin K2 ( menatetrenone) effectively prevents fracture and sustains lumbar bone mineral density in osteoporosis. J Bone Miner Res, 2000. 15: 515–521. Megj.: Bár BMD-t nem tudta növelni, de töréskockázatot kb felére csökkentette
- Iwamoto J, Takeda T, Ichinura S. Effect of combined administration of vitamin D3 and vitamin K2 on bone mineral density of the lumbar spine in postmenopausal women with osteoporosis. J Orthop Sci, 2000. 5: 546–551.
- Iwamoto J, Takeda T, Ichmura S. Treatment with vitamin D3 and/or vitamin K2 for postmenopausal osteoporosis. Keio J Med, 2003. 52: 147–150.
- Iwamoto J, Takeda T, Ichimura S. Effect of menatetrenone on bone mineral density and incidence of vertebral fractures in postmenopausal women with osteoporosis: a comparison with the effect of etidronate. J Orthop Sci. 2001; 6(6): 487–492. Megj.: Nem csökkentette töréskockázatot, BMD-t enyhén mérsékelte, de nem növelte
- Iwamoto J, Takeda T, Ishmura S. Combined treatment with K2 and bisphosphonate in postmenopausal women with osteoporosis. Yonsei Med J, 2003. 44: 751-756. Megj.: Etidronate gyógyszerrel együtt az MK-4 hatásosabban fokozta BMD-t, mint az etidronate külön. Önmagában az MK-4 nem fokozta, de segített lassítani a romlását.
- Iwamoto J. Vitamin K2 therapy for postmenopausal osteoporosis. Nutrients, 2014. 6: 1971–1980. Megj.: Etidronate gyógyszerrel együtt az MK-4 hatásosabban fokozta BMD-t, mint az etidronate külön. Önmagában az MK-4 nem fokozta, de segített lassítani a romlását.
- Ishida Y and Kawai S. Comparative efficacy of hormone replacement therapy, etidronate, calcitonin, alfacalcidol, and vitamin K in postmenopausal women with osteoporosis: The Yamaguchi Osteoporosis Prevention Study. Am J Med, 2004. 117(8): 549–555.: Megj.:Etidronatenál kevésbé, de így is kb felére csökk törés kock.
- Je SH, Joo NS, Choi B h, Kim KM, Kim BT, Park SB, Cho DY, Kim KN, Lee DJ. Vitamin K supplement along with vitamin D and calcium reduced serum concentration of undercarboxylated osteocalcin while increasing bone mineral density in Korean postmenopausal women over sixty years old. J Korean Med Sci, 2011. 26(8): 1093–1098. Megj.:Enyhe hatás 4-ből 1helyen javított BMD-n, máshol nem.
- Kanellakis S, Moschonis G, Tenta R, et al. Changes in parameters of bone metabolism in postmenopausal women following a 12-month intervention period using dairy products enriched with calcium, vitamin D, and phylloquinone (vitamin K(1)) or menaquinone-7 (vitamin K (2)): the Postmenopausal Health Study II. Calcif Tissue Int. 2012;90(4):251–262. https://doi.org/10.1007/s00223-012-9571-z. Epub 2012 Mar 4. PubMed PMID: 22392526.
- Emaus N, Gjesdal CG, Almås B, et al. Vitamin K2 (MK-7) supplementation does not influence bone loss in early menopausal women: a randomised double-blind placebo-controlled trial. Osteoporos Int. 2010;21(10):1731–1740. https://doi.org/10.1007/s00198-009-1126-4. Epub 2009 Nov 25. PubMed PMID: 19937427.
- Knapen MH, Drummen NE, Smit E, Vermeer C, Theuwissen E. Threeyear low-dose menaquinone-7 supplementation helps decrease bone loss in healthy postmenopausal women. Osteoporos Int. 2013;24(9):2499– https://doi.org/10.1007/s00198-013-2325-6. Epub 2013 Mar 23. PubMed PMID: 23525894.
- Rønn SH, Harsløf T, Pedersen SB, Langdahl BL. Vitamin K2 (menaquinone-7) prevents age-related deterioration of trabecular bone microarchitecture at the tibia in postmenopausal women. Eur J Endocrinol. 2016;175(6):541–549. Epub 2016 Sep 13. PubMed PMID: 27625301.
- Vlasschaert C, Goss CJ, Pilkey NG, McKeown S, Holden RM. Vitamin K Supplementation for the Prevention of Cardiovascular Disease: Where Is the Evidence? A Systematic Review of Controlled Trials. Nutrients. 2020;12(10):2909. Published 2020 Sep 23. doi:10.3390/nu12102909 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7598164/
- Braam LA, Hoeks AP, Brouns F, et al. Beneficial effects of vitamins D and K on the elastic properties of the vessel wall in postmenopausal women: a follow-up study. Thromb Haemost. 2004;91(2):373–380.
- Brandenburg VM, Reinartz S, Kaesler N, et al. Slower progress of aortic valve calcification with vitamin K supplementation: results from a prospective interventional proof-of-concept study. Circulation. 2017;135(21):2081–2083. https://doi.org/10.1161/CIRCULATIONAHA.116.027011. PubMed PMID: 28533322
- Shea MK, O’Donnell CJ, Hoffmann U, et al. Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am J Clin Nutr. 2009;89(6):1799–1807.
- Ikari Y, Torii S, Shioi A, Okano T. Impact of menaquinone-4 supplementation on coronary artery calcification and arterial stiffness: an open label single arm study. Nutr J. 2016;15(1):53. https://doi.org/10.1186/s12937-016-
- Knapen MH, Braam LA, Drummen NE, Bekers O, Hoeks AP, Vermeer Menaquinone-7 supplementation improves arterial stiffness in healthy postmenopausal women. A double-blind randomised clinical trial. Thromb Haemost. 2015;113(5):1135–1144. https://doi.org/10.1160/TH14-08-0675. Epub 2015 Feb 19. PubMed PMID: 25694037.
- Fulton RL, McMurdo ME, Hill A, et al. Effect of vitamin K on vascular health and physical function in older people with vascular disease—a randomised controlled trial. J Nutr Health Aging. 2016;20(3):325–333. https://doi.org/10.1007/s12603-015-0619-4. PubMed PMID: 26892582.
- Zwakenberg SR, de Jong PA, Bartstra JW, et al. The effect of menaquinone-7 supplementation on vascular calcification in patients with diabetes: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2019;110(4):883–890. https://doi.org/10.1093/ajcn/nqz147.
- Bartstra JW, Draaisma F, Zwakenberg SR, et al. Six months vitamin K treatment does not affect systemic arterial calcification or bone mineral density in diabetes mellitus 2. Eur J Nutr. 2021;60(3):1691-1699. doi:10.1007/s00394-020-02412-z
- Jamie W Bellinge, Roslyn J Francis, Sing C Lee, Nicola P Bondonno, Marc Sim, Joshua R Lewis, Gerald F Watts, Carl J Schultz, The effect of vitamin K1 on arterial calcification activity in subjects with diabetes mellitus: a post hoc analysis of a double-blind, randomized, placebo-controlled trial, The American Journal of Clinical Nutrition, 2021;, nqab306, https://doi.org/10.1093/ajcn/nqab306
- Cheung AM, Tile L, Lee Y, Tomlinson G, Hawker G, Scher J, Hu H, Vieth R, Thompson L, Jamal S, Josse R. Vitamin K supplementation in postmenopausal women with osteopenia (ECKO trial): a randomized controlled trial. PLoS Med. 2008 Oct 14;5(10):e196. doi: 10.1371/journal.pmed.0050196. Erratum in: PLoS Med. 2008 Dec;5(12):e247. PMID: 18922041; PMCID: PMC2566998.
- Lamson DW, Plaza SM. The anticancer effects of vitamin K. Altern Med Rev. 2003;8(3):303–318. Review.
- Carr BI, Wang Z, Wang M, et al. Differential effects of vitamin K1 on AFP and DCP levels in patients with unresectable HCC and in HCC cell lines. Dig Dis Sci. 2010.
- Mizuta T, Ozaki I, Eguchi Y, et al. The effect of menatetrenone, a vitamin K2 analog, on disease recurrence and survival in patients with hepatocellular carcinoma after curative treatment: a pilot study. Cancer. 2006;106(4):867–872.]
- Abe Y, Muta K, Hirase N, et al. Vitamin K2 therapy for myelodysplastic syndrome. Rinsho Ketsueki. 2002;43(2):117–121